Consumer awareness of evolving new ingredients and technologies for skin care continues to expand, and some ingredients have changed the scope of modern skin treatment products—the introduction of alpha and beta hydroxy acids and retinoic acid, for example. The blend of these ingredients with others to address, among other applications, antiaging, hyperpigmentation and acne concerns and problems brought about the age of cosmeceuticals.
As part of this age, chiral skin care appeared via several niche professional brands in the late 1990s. It coincided with the growing employment of chiral technology by the pharmaceutical industry. Skin care has been slower to embrace chiral technology, largely because the concept is more difficult for consumers to understand. However, as skin is the largest organ of the body, the imperative for chiral assessment in skin care is inevitable. The recognition of the biological importance of chiral ingredients and their applications in skin care and other topical products will be explored, and specialty skin care brand owners may uncover new opportunities in the information—along with new business opportunities and partnerships with their product development teams, ingredient suppliers and clinical testing laboratories.
What is Chirality?
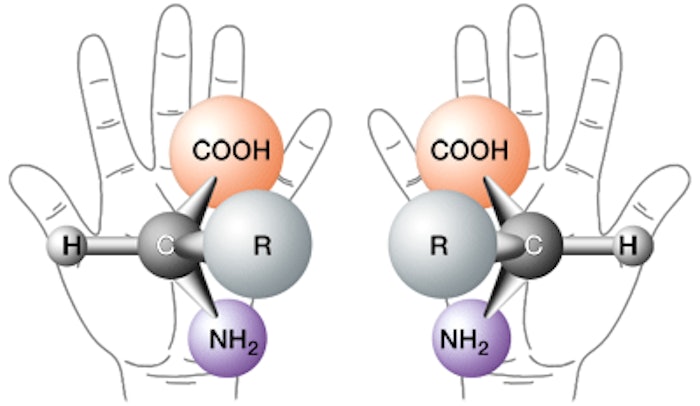
All organic natural materials must contain carbon atoms, and each carbon atom has four hands arranged in a three-dimensional manner. Two hands (solid lines) are on the sides, one is in the front (heavy line) and one is in the back (broken line).
Each hand must be connected to another hand, which can be another carbon (C), and/or an element selected predominantly from hydrogen (H), oxygen (O) and nitrogen (N). Sugars, carbohydrates and hydroxy acids contain C, H, and O; amino acids and proteins contain C, H, O, and N, for example. Sulfur (S) and phosphorus (P) atoms are present in lesser number of natural compounds.
If at least two elements attached to any single carbon atom are identical, these organic materials are called achiral. Again, illustrated in Figure 1. If none of the elements attached to any single carbon atom are identical, the organic materials are called chiral.
All organic materials are either achiral or chiral, and an organic material can contain more than one chiral carbon atom. However, note that these organic materials can also be synthetic—and they, too, are thus either achiral or chiral (see Nature Identical).
Mother Nature is a Chemist
Molecules produced in nature are mostly chiral, and chiral molecules play a critical role in the biology of all living matter. Mother Nature is also three-dimensional and left-handed.
Again, when an organic compound contains at least one carbon atom (asymmetric carbon) to which those four atoms attached are different in chemical nature, then that molecule is chiral, and these molecules are left-handed or right-handed—depending on the orientation of those four atoms attached to the asymmetric carbon.
Among popular methods of nomenclature, chiral molecules are designated D or L; chiral atoms are labeled R or S. Chiral molecules have a unique physical property of deflecting a beam of polarized light to either the left or the right side of a centerline. However, a left-handed molecule, for example, may deflect polarized light to either the left or the right side of that centerline. The direction of that rotation is not directly related to either left- or right-handedness of a chiral molecule. The direction of light deflection is designated (+) for right-handed (d) and (-) for left-handed (l) rotation. The left-handed and right-handed molecules usually have different physical, chemical and biological properties, and the pharmaceuticals industry has utilized this variance in biological properties of chiral ingredients, both natural and synthetic, in the treatment of human ailments for a long time. Amoxicillin, a commonly prescribed penicillin derivative, is a good example of a chiral molecule and the importance of the orientation of its carbon molecules has the chemical name: (2S,5R,6R)-6-[(R)-(-)-2-amino-2-(p-hydroxyphenyl) acetamido]-3, 3- dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0] heptane-2-carboxylic acid. Note the chiral molecules labeled with “S” and “R.” In this case, the change of orientation of any of the carbons labeled as such usually leads to the total loss of the biological activity.
Chirality All Around You—Consider the Applications
Chiral molecules exist everywhere. Your own DNA is chiral—as are your hormones, enzymes and the structural components of tissue, blood, hair and skin. Consider, too, the building blocks of proteins—amino acids. They are thought to be the first organic molecules produced on Earth via abiogenesis (the primordial soup theory), and all amino acids are chiral, with the exception glycine.
The applications of chiral ingredients in skin care and other topical products are also abundant.
Before discussing how these ingredients work, a quick analogy: People are either right-handed or left-handed. Right-handed people work better with their right hand; left-handed people work better with their left hand. Very few people are ambidextrous, working equally well with their right or left hand.
Just like people, these right-sided or left-sided natural ingredients perform uniquely in many cases—as previously touched upon in the amoxicillin example. A left-handed ingredient can have a greater skin wrinkle reduction benefit than a right-handed ingredient—ascorbic acid being a prime example. An L ingredient can be skin soothing, while the same ingredient in its D-form may cause skin irritation. If skin irritation property of a D-form is greater than skin soothing property of an L-form, then the DL-form may cause skin irritation overall. The use of pure 100% L form in such cases, for example, is essential.
Natural ingredients included in a cosmetic product that are either L or D, too, may not absorb into skin at the same rate. If an L ingredient is absorbed more rapidly, then a 50:50 mixture of both L and D ingredients may have less beneficial effects compared to the use of a 100% pure L ingredient. If the ingredient has been made via synthesis in a pure L- or D-form, then both the natural and the synthetic would provide the same and equal biological properties. Synthetic ingredients are not bad, as long as they are nature-equivalent and have no undesirable chemical impurities. Examples follow.
Chirality in ascorbic acid: Ascorbic acid is a sugar acid possessing two chiral carbon atoms. The L-form of ascorbic acid is commonly known as vitamin C. A recent study has shown that D- and L-forms of ascorbic acid have different affinity for the enzyme tyrosinase, which catalyzes the production of melanin and other pigments. This would indicate that the skin-whitening efficacy of L-ascorbic acid and D-ascorbic acid are vastly different. The efficacy of protective effects of D- and L- ascorbic acid on light-induced retinal damage in rats in one study showed vastly different biological efficacy of these isomers. In addition, 5-lipoxigenase, an enzyme causing cellular oxidative damage, is selectively inhibited by L-ascorbic acid; D-ascorbic acid reverses this beneficial effect.
Chirality in alpha-tocopherol (vitamin E): There are eight isomers of alpha-tocopherol that differ in the arrangement of groups around those chiran centers. Natural alpha-tocopherol is the D-alpha form, and the synthetic form (DL-alpha) is not as active as the natural tocopherol form. Therefore, the synthetic DL-alpha-tocopherol probably has only about half the vitamin activity of natural D-tocopherol in humans.
Chirality in lipoic acid: There is a carbon atom in lipoic acid that is chiral and the molecule exists as two enantiomers (a left-hand and a right-hand)—R and S—that are pharmacologically distinct. Only the R occurs naturally. Lipoic acid has been found to increase the production of glutathione, responsible for glucose uptake in cells, and R has been shown to do be more effective than S or a mixture of the two.
Chirality in amino acids: Of the 23 alpha-amino acids important in human biology, all but one (glycine) are chiral. These amino acids play a vital role in natural skin care, the production of skin and hair protective colorant (melanin), urocanic acid (sunscreen), cellular turnover and protein synthesis are among those roles. L-tyrosine and L-dopa amino acids are used in hair-darkening formulations, and the inhibition of L-tyrosine and L-dopa synthesis (tyrosinase inhibitors) is used for skin-whitening applications.
As brand owners and their partners push the technology envelope for beauty, recognizing the biological importance of chiral ingredients and their applications is a good foundation for uncovering opportunities, efficiency to market and making an impact with consumers.
Shyam Gupta is a consultant in skin and hair care ingredients and topical delivery systems. He specializes in nature-and science based formulations with enhanced efficacy and consumer desirable performance attributes. 1-602-996-9700; [email protected]; www.biodermresearch.com
Linda Walker is the president of CoValence Laboratories, a premier skin care manufacturer and originator of chirally correct skin care products. 1-480-897-0551; [email protected]; www.covalence.com










