View this article and others in the June 2020 digital magazine.
Dynamics in the hair care segment create numerous opportunities and formulation challenges for hair and scalp care. The global hair care market is expected to amount to US $102 billion by 2024, suggesting a lucrative market for innovative solutions. In response, cationic encapsulation microsystems (CEMs) were developed and are proposed here for their ability to meet and address current hair market trends and concerns.
The present article describes the characteristics of CEMs and brief history, along with differences in hair biology and how it reacts to environmental damage. CEM systems are then proposed to develop specialized hair care formulations, such as for heat and color protection, the latter of which is explored in greater depth in a shampoo test for hair color-retention capabilities.
Cationic Encapsulated Microsystems
Cationic Encapsulated Microsystems (CEMS) are lipid micro dispersions composed of one or more concentric layers of phospholipids entrapping an aqueous phase (see Figure 1). They can be designed to transport hydrophobic molecules in the lipid membrane and hydrophilic substances in the aqueous phase. Depending on their structure, they can interact with cells of the body by fusion, endocytosis, adsorption and transference of lipids from the phospholipid membrane.
Micro dispersions are a next-generation technology of early research conducted by Alec Bangham, who is credited as the first person to describe similar structures—liposomes, in 1964. Initially these materials were referred to as liquid crystals, although Bingham called them “Bangasomes,” describing their chemical and physical nature in the Journal of Molecular Biology;1 later, they became commonly referred as liposomes. In 1971, medical researchers evaluated the use of liposomes for drug delivery.2, 3 Since the formation of liposomes does not require potentially irritating surfactants or emulsifiers, they are highly desirable for skin and hair care use.
Variations in Fibers and Follicles
As is well-known, to some extent, everyone’s hair is unique in its production rate, size and shape. Genetic factors account for many of these differences. Curly or straight hair, color and cross-sectional diameter of the hair fiber also differ across ethnicities. While proteins, keratins and molecular structure have common characteristics within ethnicities, the physical characteristics of hair may react differently from exposure to the environment and hair treatments.4
Three distinct anatomical regions are associated with hair: the cuticle, the cortex and the medulla. The cuticle is the outermost layer of hair, protecting hair from environmental insult. It is composed of flattened scale-like cells that overlap each other. These scales slope outward from their attachment on the cortex, and the free ends of the scales interlock with the cells of the inner root sheath to hold the hair in the follicle. The cortex is the main body of the hair and is composed of elongated and spindle cells. It contains pigment granules—structures that primarily give hair its characteristic color. The organization, density, size and distribution of these pigment granules varies between racial groups and even individuals.
Keratin is the main component of scalp hair. It contains all 21 amino acids, although the ratio of keratin amino acids may vary to some degree depending upon the individual’s biochemistry. Lipids in hair are composed of fatty acids, cholesterol sulfate, ceramides and cholesterol. These lipids provide a protective barrier for hair. This lipid composition appears to be similar in content among people, unlike the lipid composition of the epidermis.4, 5
Variations in Response to Environment
Ji et al.4 studied the effects of UV exposure and hair damage at 12 hr and 48 hr after UVA (20 J/sec) and UVB (8 J/sec) irradiation. Changes in hair lipids were evaluated using scanning electronic microscopy (SEM) and transmission electron microscopy. African hair showed the highest amount of surface damage, compared with Caucasian and Asian hair.
While lipid composition initially was similar across all ethnic hair types, free fatty acids content was higher in Asian hair. After UV irradiation, lipid content was decreased in all hair types. Also after UV irradiation, European and African hair exhibited more damage, compared with Asian hair.
The investigators thus concluded that integral hair lipids may protect the hair against UV light. They evaluated the effect of UV on the hair cuticles of the three ethnic groups. All groups had intact and tightly overlapping cuticle scales. With increased UV radiation, i.e., UVB and UVA radiation damage measured at 12 hr and 48 hr exposure, the surface of all cuticles became increasingly damaged. UVB resulted in more severe damage than UVA, and African hair showed the most severe damage.
Absorption is followed by swelling, and both are essentially related to pH. Swelling favored by alkaline pH and polar solvents, including urea solutions, acetamide and lithium bromide, promote hair swelling as well.
Evaluation of the cuticle damage by transmission electron microscopy showed that as irradiation energy increased, variably sized holes and cleavage developed along the endocuticles of the hair. All three hair groups exhibited similar damage from both UVB and UVA exposure.4
Hair threads are natural fibers consisting of approx. 65-95% of hair’s weight, composed primarily of keratin proteins. The proteins have a high concentration of sulfur associated with the amino acid cystine.5, 6 In relation, the physical properties of hair mainly depend on the hair geometry. Caucasian hair is oval, Asian hair is circular and African hair is elliptical. Elasticity, smoothness, volume, degree of shine and softness of hair are related to the condition of the cuticle scales.6, 7 The condition of the cuticle and adherence to the hair fiber also has an impact on the ease of combing.
With respect to hair care, the cuticle is the most important component because it is most affected by cosmetic treatment. Products including conditioners, hair sprays, mousses and gels deposit on the cuticle. Hair dryers, hair straighteners and curling products all traverse and permeate through the 8 to 11 layers of cuticle that overlap each other to ultimately impact the appearance and condition of the hair fiber.6
Interestingly, the weight needed to rupture a natural hair thread is approximately 50 g to 100 g. The average human head has about 120,00 threads of hair and would theoretically support 12 tons.6 The resistance to breakage is a function of the diameter of the hair thread and condition of the cortex; chemical treatments, of course, can weaken the hair fiber. Hair fibers have elastic properties and may undergo moderate stretching when wet or dry. When dry, the hair thread may stretch to 20-30% of its length whereas when wet, it may reach 50%.6, 7 Again, chemical and physical treatments, sun exposure, and the use of electric dryers and heated irons typically impact hair stretching.
Hair absorbs water; in fact, keratin may absorb up to 40% of its own weight in water. Hydration is promoted by temperature increase, pH and polar solvents that break hydrogen bonds and alter the properties of hair, including stretching and hair diameter.8, 9 Absorption is followed by swelling, and both are essentially related to pH. Swelling favored by alkaline pH and polar solvents, including urea solutions, acetamide and lithium bromide, promote hair swelling as well.8, 9
Combing hair produces an electric load generated by friction and electric resistance of the hair. The resulting static load is a function of the hair fiber conductivity or electrical resistance. Long chain quaternary ammonium salts increase conductivity on the hair thread surface and reduce friction.
Interestingly, in an electric load test, Syed et al.10 found that African hair developed a high negative static load (-25 kV/m), whereas Caucasian hair developed a low positive static load (+6.6 kV/m). Syed hypothesized the high negative static load in African hair was the result of the additional force necessary to run the comb through the hair;10 notably, high electrostatic loads may promote hair bristling.11
CEM Design and Properties
The CEMs described here, as stated previously, are vesicles composed of one or more concentric layers of phospholipids entrapping aqueous phases. They consist of purified phospholipids from soy lecithin and are predominantly phosphatidylcholine with low levels of impurities. An average particle size between 110 nm and 300 nm in diameter ensures their long-term stability. The physical characteristics of CEMs are influenced by the lipid composition used and the method of preparation—here, a specialized micro fluidization manufacturing process ensures their replicable particle size, stability and high encapsulation efficacy of functional materials.
Also, considering wet hair has a negative charge, these CEMs were designed to have a positive charge, facilitating strong adhesion to the hair fiber surface (see Figure 2). Indeed, it is not only composition and particle size, but also surface charge that ensures their efficacy.1, 2 This charge is known as the zeta potential; i.e., the overall charge a particle acquires in a specific medium. It can be used to indicate how CEMs will function with respect to a desired application. The most convenient method to determine electrostatic properties is through the determination of the zeta potential of an ensemble of charged lipid vesicles in an external electric field. Thus, particle size, charge and zeta potential are key to ensuring CEMs designed for hair care can penetrate the damaged hair, ultimately to prevent lipid loss (see Figure 3).
While proteins, keratins and molecular structure have common characteristics within ethnicities, the physical characteristics of hair may react differently from exposure to the environment and hair treatments.
Thermal Protection
Previously, a CEM encapsulating phospholipids, polyquaternium-16, phenyl trimethicone, Prunus amygdalus dulcis oil, hydrolyzed silk, panthenol and tocopherol was designed to protect hair fiber from sudden temperature changes, especially when using heated hair straighteners and hair driers. It was shown to form a protective film to decrease heat transfer speed to hair, preventing vapor bubbles that damage the hair structure (see Figure 4; data not shown). It also lubricated and conditioned the hair fiber, improving its condition after treatments that typically would damage hair (see Figure 5; data not shown).
Color Fixation and Protection
For the present study, a different CEM was designed containing phospholipids, polyquaternium-16, dimethicone and tocopherol acetate to condition and brighten hair, and provide volume and elasticity. Previously, this CEM was shown to improve hair condition; i.e., resistance to traction and minimizing hair loss resulting from breakage by combing (see Figure 6; data not shown). Here, it was added to oxidative dyes and its effects were assessed for color fixation and color fade prevention by successive hair washing (Figure 7a). The following test protocol was utilized.
Materials and Methods
As stated, the objective of this study was to measure how a test shampoo containing (or not; control) the CEM affected the amount of dye released from color-treated strands of hair after several wash cycles.
Hair preparation: Natural virgin hair was divided into five strands weighing 5 g each. Four were color-treated; the control strand was not colored. The test strands were treated with 70 g of bleach powder of hydrogen peroxide solution, long enough for all the hair to be completely discolored.
Hair was then dyeda; 5 g of dye was added to 7.5 mL of hydrogen peroxide (20 volumes), stirred to a uniform mixture, then applied to the bleached strands. The mixture remained on hair for 40 min, then rinsed with warm water to remove the remaining coloring cream. Washing was carried out using 0.50 cc of base shampoo to remove remaining coloring residue. Hair was then dried with a hot air dryer on medium power for 1 min. The amount of dye attached to the capillary fiber of the hair strands was quantified by FTIR by means of diffuse reflectance methodology, described in the next section (see Figure 7a).
Shampoo protocol: The color-treated hair strands were next washed for 10 cycles with warm water and 0.5 cc of one of five test shampoos, as follows:
- No shampoo (control)
- Base shampoo (see Formula 1)
- Base shampoo containing 0.50% CEM
- Base shampoo containing 1.00% CEM
- Base shampoo containing 2.00% CEM
The shampoo was massaged into hair with both hands for 1 min along the entire strand, then rinsed with warm water. The strands were dried using a towel, followed with a hot air dryer at moderate temperature for 1 min. Photographs were taken of all the strands to visually assess the initial fixation of the color in the original strands, and color changes after 10 successive wash cycles (see Figure 7b).
Scanning electron microscopy (SEM): SEM was used to visibly evaluate the appearance of treated hair strands compared with the control.
Infrared Fourier Transform Spectroscopy Diffuse Reflectance (DRIFTS): DRIFTS was used to assess the percentage of color retained after shampooing. This approach collects and analyzes scattered infrared (IR) energy. It is used for the measurement of fine particles, powders and rough surfaces. When the IR beam enters a sample, it can either be reflected off the surface of a particle or transmitted through it. The IR energy reflecting off the surface is usually lost. The IR beam that passes through can, again, either be reflected off of or transmitted through the next particle. The scattered IR energy is collected by a spherical mirror that is focused onto a detector. Detected light is partially absorbed by particles of the sample, and readings are generated as shown (see Figure 8).
Once attached to the hair, the CEM retains the encapsulated “active” substances in place to impart their effects, preventing them from being eliminated when the hair is rinsed.
This FTIR generated spectrum shows spectra labeled AMIDE I and AMIDE II. These are specific peptide bonds that comprise keratin proteins. Oxidative dyes form compounds that contain functional groups that vibrate in the AMIDE I and AMIDE II areas. The spectrum associated with the specific dye used show the heightened peaks in the spectra.
The spectrum shows the carbonyl group C=O vibrates in the AMIDE I zone, and the bonds C-N-H are stretched and deformed in the AMIDE II area (see Figure 9). For bleached hair, the spectrum area of AMIDE I and AMIDE II are relevant to show the direct reflection of the vibrations and deformations of the functional groups of the amino acids that comprise capillary protein (keratin). In dyed hair, the peaks represent the contribution of the amino acids that constitute the capillary fiber, together with the contribution of the functional groups of the coloration.
Previous studies have shown that bleached hair registers higher peaks in the regions of AMIDE I and AMIDE II in a proportional manner. The heights of these peaks can be quantified using software in the FTIR unit. Only contributions of the amide groups from the capillary proteins in hair strands and hair coloration are measured.
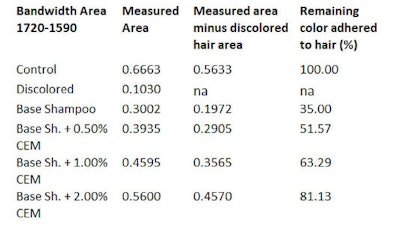
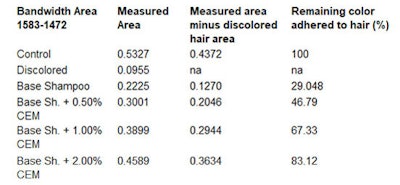
By subtracting the area of the bleached hair, the resulting measurable area will belong to the dye. To calculate the percentage of color release during 10 wash cycles, the amount of excess colorant released during the first wash cycle of the dyed hair strands is considered as 100% (see Table 1, Table 2 and Table 3).
Taken together, images from scanning electronic microscopy and FTIR by diffuse reflectance give an assessment of visible damage to the tested hair strands and a quantification of color loss after shampoo treatment with and without the cationic encapsulation microsystem treatment.
Results and Conclusions
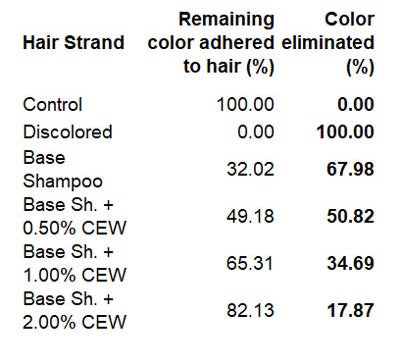
The dyed hair strand washed with the base shampoo without CEM for 10 cycles lost 67.98% of its original color (see Table 3). The dyed hair strand treated with the shampoo including 0.5% CEM lost approx. ~51.0% of its original color. The dyed hair treated with 1.0% and 2.0% CEW shampoo and washed for 10 cycles lost only about 35% and 18.0% of its original color, respectively. Most striking, however, was the damage caused by the base shampoo without CEM after 10 wash cycles (see Figure 10 and Figure 11).
Based on the findings presented, it appears the CEMs are attracted to hair and effectively deliver encapsulated components specifically to damaged areas from the final formulation. Once attached to the hair, the CEM retains the encapsulated “active” substances in place to impart their effects, preventing them from being eliminated when the hair is rinsed.
References
- Bangham, A.D. and Horne, R.W. (1964). Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J Mol Biol 8(5) 660-668.
- Gregoriadis, G. and Ryman, B.E., (1971). Enzyme entrapment in liposomes. FEBS Letters 14(2) 95-99.
- Patzelt, A. and Lademann, J. (2013, Jun). Drug delivery to hair follicles. Expert Opinion on Drug Delivery 10(6) 787-797.
- Ji, J.H., Park, T.-S., … Lee, W.-S., et al. (2013). The ethnic differences of the damage of hair and integral hair lipid after ultraviolet radiation. Ann Dermatol 25(1) 54-60.
- Lee, W.-S., Oh, T.H., … Hwang, S., et al. (2005, Dec). Integral lipid in human hair follicle. Soc Invest Derm 10(3) 234-237.
- Velasco, M.V.R., de Sa Dias, T.C., … Baby, A.R., et al. (2009). Hair fiber characteristics and methods to evaluate hair physical and mechanical properties. Brazilian Jour Pharm Sci 45(1) 153-162.
- Juez, J.L. and Gimier, L. (1983). Ciencia Cosmética, 2nd edn. Madrid: Soc Espanhola de Quim Cosmet p 98-119.
- Robbins, C.R. (1997). Chemical and Physical Behavior of Human Hair, 3rd edn. New York: Springer.
- Feughelman, M. (1997). Morphology and properties of hair. In: Johnson, D.H., ed, Hair and Hair Care. New York: Marcel Dekker 1-32.
- Syed, A.N., Kuhajda, A., Ayoub, H., Ahmad, K. and Frank, E.E. (1996). Cabelo Afro-Americano vs. Caucasiano: Propriedades. Cosmet Toiletries Brasil 8(3) 55-59.
- Robbins, C.R. and Crawford, R.J. (1991) Cuticle damage and the tensile properties of human hair. J Soc Cosmet Chem 42(1) 59-67.